Vision
To assist with the development and customization of individually formulated medicinal products (IFMPs)
Our standards
We apply the highest standards for manufacturing and testing:
A) OTC products, often marketed as dietary supplements: ISO 9001, HACCP, ISO 17025 (accreditation for the testing),
B) medicinal substances and formulations that have the highest quality requirements of pharmacopoeias (e.g. ČL (Czech Pharmacopoeia), DAB (Deutsches Arzneibuch), Ph. Eur. (European Pharmacopoeia)), and other legislation, often individual to EU countries, to achieve EU-GMP quality.
Overall, we hold these certifications by the SÚKL (State institute for drug control), for medical cannabis:
- testing (GMP, VYR-32),
- manufacture (GMP VYR 26),
- distribution (GMP, GDP).
NOTE: the great popularity of cannabis in general requires the consistent application of the necessary standards. However, many of these are not universally accepted, so it is essential to communicate local requirements very well. Furthermore, regulatory measures in terms of psychotropic compounds, especially THC, need to be respected.
Our production rules
- We strictly implement any production in a legal manner. We do not carry out production or testing without demonstrable proof of production.
- As a rule, we rely on our own plant sources. Where clients have quality plant sources, these may be used but must meet quality specifications, logistics conditions and minimum quantities.
- We very consistently meet the requirements of regulatory authorities and certification bodies such as local FDAs, especially where we work in the medicinal cannabis field.
- We respect the requirements of DG SANTE for the Novel Foods Directive, although we are not without reservations and believe in a positive shift in its restrictive nature. However, we have implemented the relevant registration steps.
- In the absence of standards, we have brought many of our own to validate. We reserve the right to maintain appropriate confidentiality about their details. These procedures do not automatically transfer to ownership of the products produced.
- We believe that the maximum effect of plants, including cannabis, comes from their overall composition. Therefore, we prefer, and recommend to clients, full spectrum products.
- We switch to isolated forms of active ingredients when warranted.
- We combine our manufacturing processes exclusively with our own analytical procedures. Tests carried out by third parties are respected but there are indicative for us in the end.
ISO 9001/HACCP production scheme
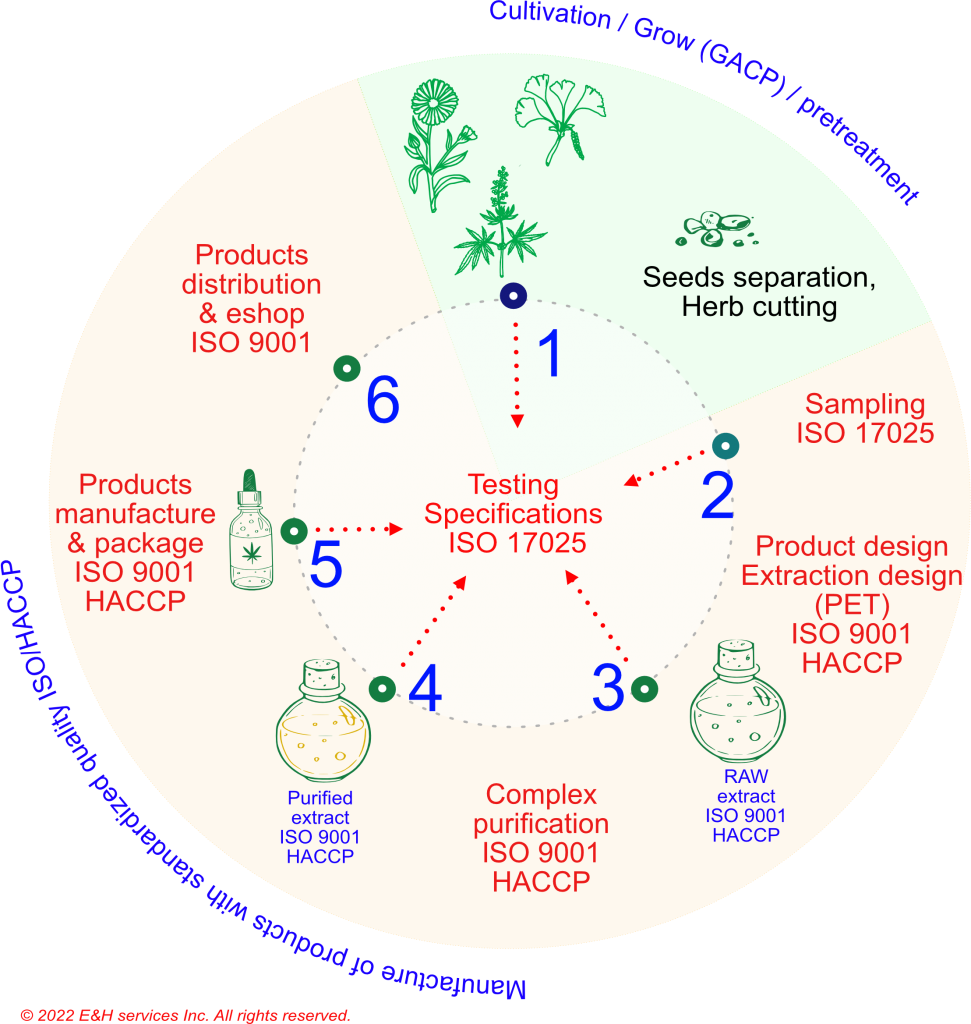
- Input of the raw material to be grown. The raw material is dried; it is stripped of its seeds, and part of the plant (often the flowers) is cut to the optimum fraction size. The treated raw material is stored in a safe manner (bags, boxes) and stored in a dry place.
- The quality of the raw material is tested, the essential procedure being the selection of a representative sample. This is the basis for the pilot production of the crude extract.
- The raw extract (RA) is the input to the optimal composition of the product portfolio from a specific plant.
- 80% of the crude extracts (RA) must be comprehensively purified. Without this process, PAHs, PCBs, PCDD/Fs, OCPs and other similar substances, often of a non-polar nature, may be present in the extract, especially if the active ingredients are preserved in a non-polar resin.
The most common contamination is PAH and PCDD/F (for plants grown as “outdoor”), and we have confirmed cases of PCB or OCP contamination.
- Our purified extracts (PE) are the basis for the final production of high quality products.
- Manufactured products are filled into suitable packaging, packaged and distributed to end clients (e.g. via eShop) or handed over for the client’s own distribution.
GMP production of medicinal cannabis substances (extracts)
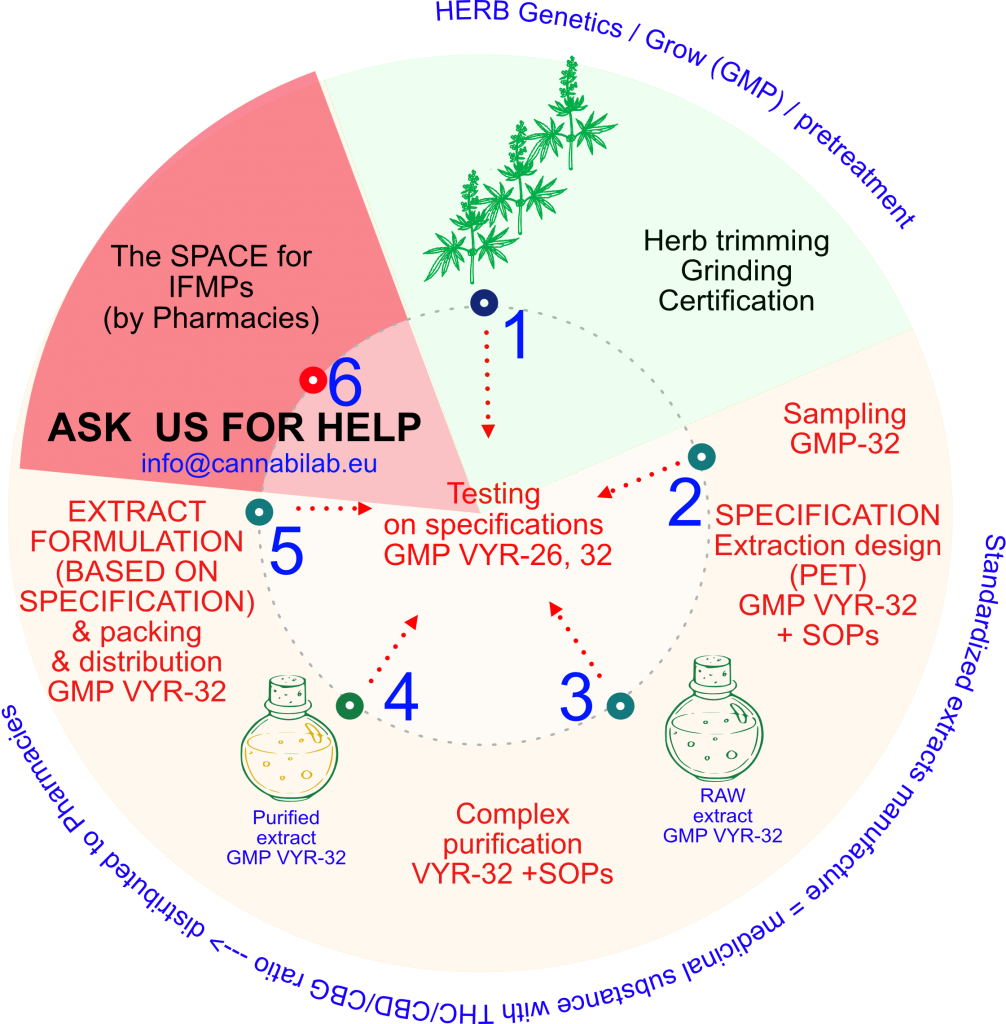
1) Input of quality raw material, GMP quality. Dried raw material is used, which, if not, is adjusted to the size of the optimal fraction.
2) The quality of the raw material is tested in GMP mode, according to established specifications.
3) Raw extract (RA) is produced by a validated process.
4) The crude extract is purified in several steps. The degree of purification is selected according to the desired quality/specification of the standardized extract.
5) The purified extract is prepared for the manufacture of pharmaceutical products, in IFMP mode.
6) The IFMPs (individually formulated medicinal products) are generally manufactured in pharmacies. Many of them require appropriate development and validation, in which we are ready to help.
Need to help or your interest?
Contact us to info@cannabilab.eu.
